
The US FDA New Drug Approvals in November 2024
Shots:
-
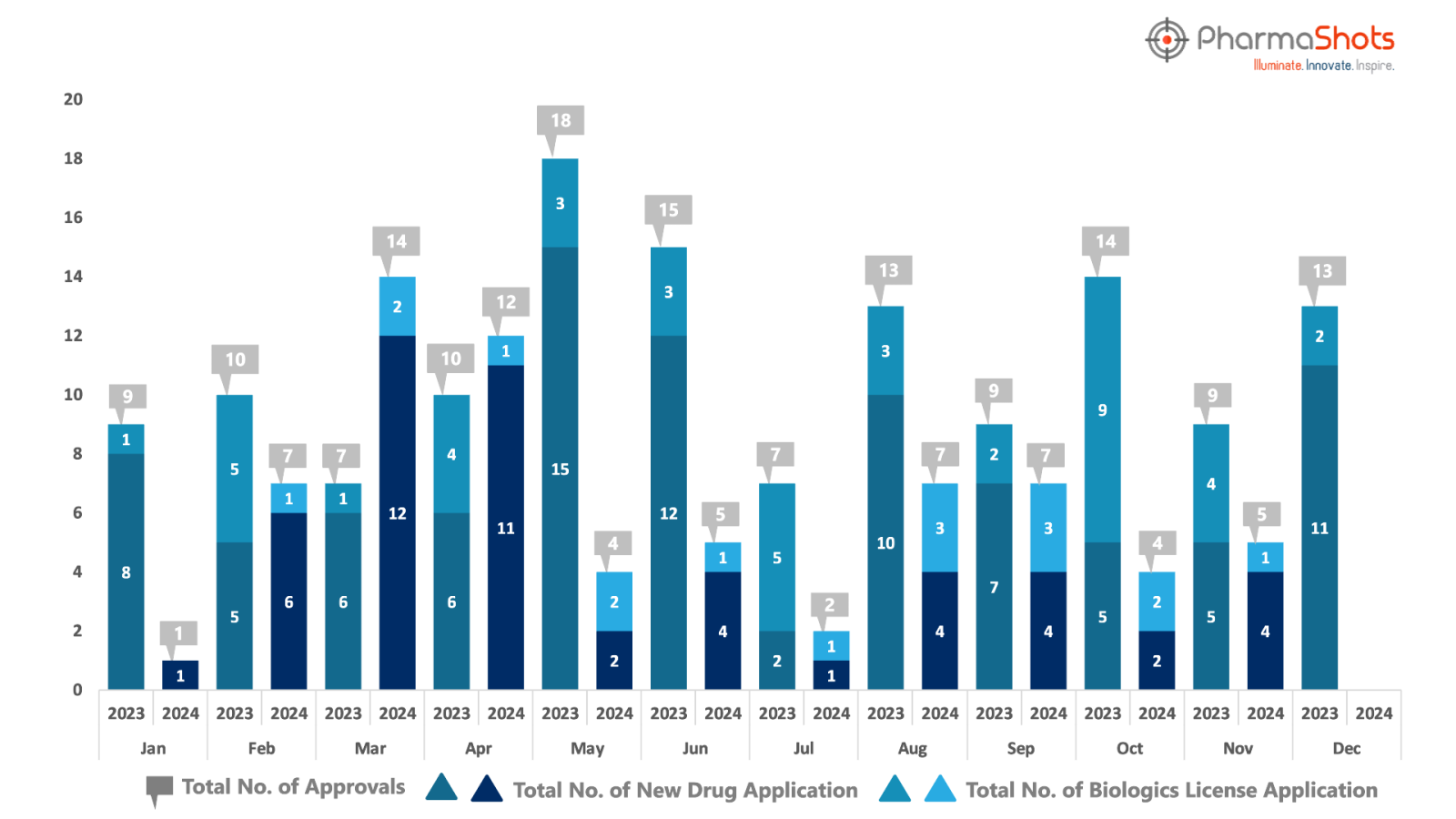
PharmaShots has compiled a list of US FDA-approved drugs in the month of November 2024
-
The US FDA has approved a total of 5 new drugs including 4 new molecular entities and 1 biologic leading to the treatment of patients and advances in the healthcare industry
-
The major highlighted drug was Jazz Pharmaceuticals’ Ziihera securing accelerated approval for treating HER2+ Biliary Tract Cancer
1. Syndax Pharmaceuticals’ Revuforj (Revumenib) Receives the US FDA’s Approval for R/R Acute Leukemia
Product Name: Revuforj
Active ingredient: Revumenib
Company: Syndax Pharmaceuticals
Disease: Relapsed/Refractory Acute Leukemia
Date: Nov 15, 2024
Shots:
-
Based on P-I/II (AUGMENT-101) study, the US FDA has approved Revuforj to treat r/r acute leukemia with a lysine methyltransferase 2A gene (KMT2A) translocation in individuals (age≥1yr.)
-
In 104 efficacy evaluable patients, data showed CR + CRh was 21%, with median duration of 6.4mos. & median time to CR or CRh of 1.9mos.; 23% underwent HSCT post Revuforj.
-
The company will release 110 & 160mg tablets in Nov across the US and 25mg tablets for patients with wt. <40kg in H1’25. An oral formulation will available under expanded access program for those with <40kg before the availability of 25mg tablets
Product Name: Ziihera
Active ingredient: Zanidatamab-hrii
Company: Jazz Pharmaceuticals
Disease: Biliary Tract Cancer
Date: Nov 20, 2024
Shots:
-
The US FDA’s accelerated approval of Ziihera (50mg/mL, IV) was based on P-IIb (HERIZON-BTC-01) study in patients (n=87) with HER2+, locally advanced unresectable or metastatic BTC across 2 arms, based on tumor IHC status
-
Efficacy in 62 HER2+ BTC patients (arm 1 of HERIZON-BTC-01) showed a 52% ORR & 14.9mos. mDoR. Continued approval depends on P-III (HERIZON-BTC-302) confirmatory trial of zanidatamab + SoC vs SoC alone in 1L HER2+ BTC
-
Zanidatamab is also being studied P-III (HERIZON-GEA-01) trial with CT & with/without tislelizumab for 1L HER2+ GEAs as well as P-III (EmpowHER-303) trial with physician's choice CT HER2+ mBC patients, progressed on or intolerant to trastuzumab deruxtecan
Product Name: Rapiblyk
Active ingredient: landiolol
Company: AOP Health
Disease: Atrial Fibrillation & Atrial Flutter
Date: Nov 22, 2024
Shots:
-
The US FDA has granted approval to Rapiblyk for treating severe heart condition supraventricular tachycardia (atrial fibrillation and atrial flutter) in critical care setting
-
Approval was supported by 5 clinical trials assessing the safety & efficacy of Rapiblyk (9.3 to 74.6 mcg/kg/min) vs PBO in adults (n=317) with supraventricular tachycardia, showing 40-90% vs 0-11% reduction in the heart rate & 9.9% vs 1% AEs
-
Rapiblyk, adrenergic receptor antagonist with beta 1/2 selectivity, lowers heart rate without affecting blood pressure. It is approved in the EU for treating supraventricular tachycardia, incl. atrial fibrillation & flutter, and managing non-compensatory sinus tachycardia
4. BridgeBio Pharma’s Attruby (Acoramidis) Secures the US FDA’s Approval to Treat ATTR-CM
Product Name: Attruby
Active ingredient: Acoramidis
Company: BridgeBio Pharma
Disease: Transthyretin Amyloid Cardiomyopathy
Date: Nov 22, 2024
Shots:
-
The US FDA has approved Attruby (oral stabilizer of Transthyretin) to reduce cardiovascular death & associated hospitalization in ATTR-CM patients. The MAA is also under review in the EU, with decision anticipated in 2025
-
Approval was based on P-III (ATTRibute-CM) study of Attruby vs PBO in ATTR-CM patients (n=632). It achieved its 1EP, showing a 42% reduced ACM & recurrent CVH events, 50% reduced cumulative frequency of CVH events at 30mos. & a Win Ratio of 1.8 for a composite of ACM, CVH, NT-proBNP & 6-minute walk distance
-
Bayer holds exclusive commercial rights of Attruby in the EU & will get $500M under their royalty agreement
Note:
According to the FDA's November 2024 approval list, the following drug was also approved; however, no PR was available:
-
Iomervu
Related Post: Insights+: The US FDA New Drug Approvals in October 2024
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














